
24-01-2026
What Is Benzene? A Guide to Its Characteristics & Byproducts
When we are talking about aromatic hydrocarbons, you might often find benzene, especially in a manufacturing process. Benzene is the simplest aromatic hydrocarbon and is widely used in the chemical industry. In fact, it becomes the starter stock for producing other chemical compounds.
This article delves into the intricacies of benzene, encompassing its definition and applications. So, read the complete information below!
What Is Benzene?
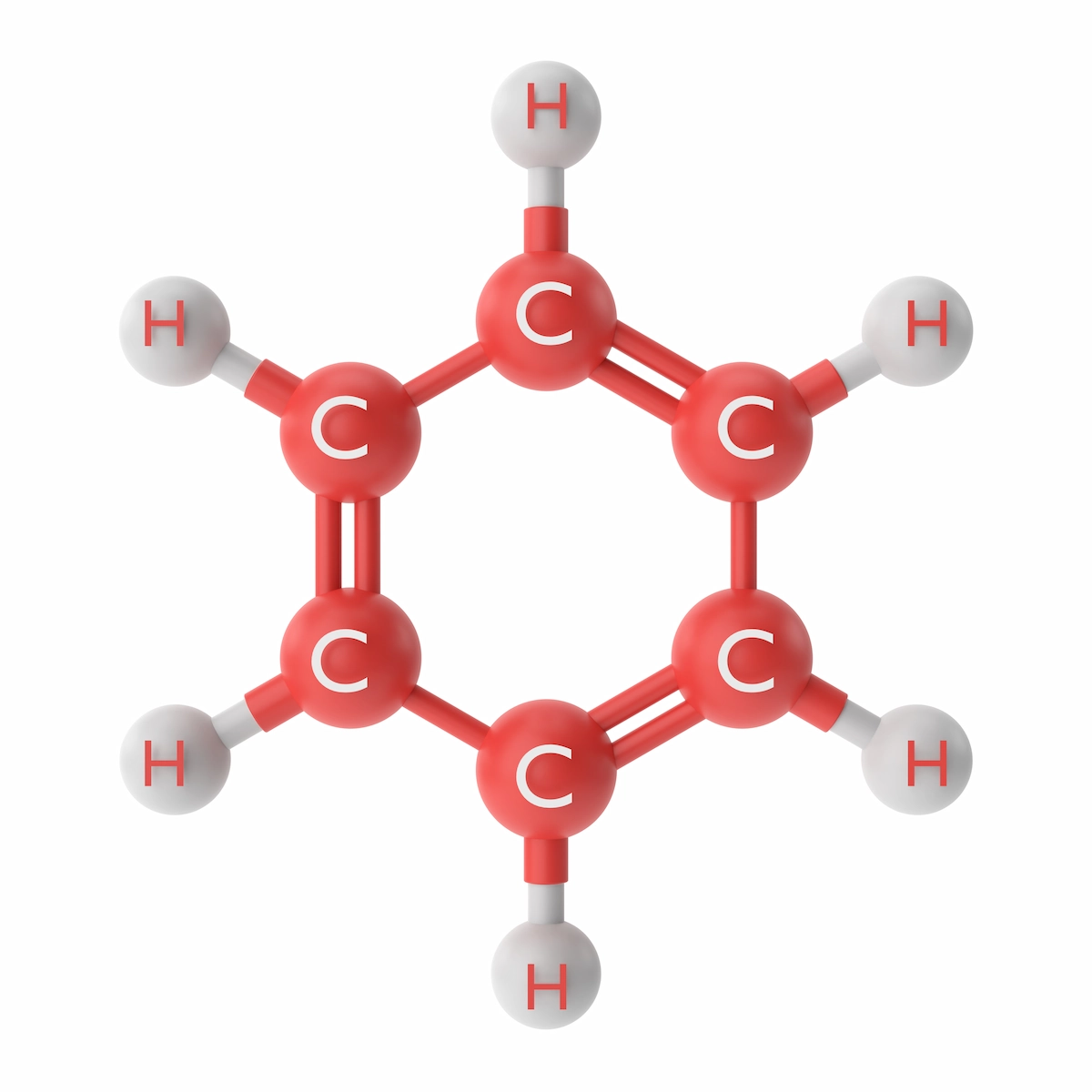
Benzene is an aromatic hydrocarbon having ring-like molecular structures. This ring contains six carbon atoms and six hydrogen atoms, constructing a formula of C6H6.
You can find benzene in gasoline, natural gas, and crude oil. It becomes the builder component for chemicals used in the industry, such as detergents, solvents, pesticides, and plastics. Some examples of benzene’s byproducts are cumene, cyclohexane, and ethylbenzene.
Benzene has several characteristics, such as being colorless or light yellow, having a sweet aroma, being volatile, and having a vapor that is heavier than air. When it is combusted, benzene emits pitch-black smoke.
The common uses of benzene are becoming the feedstock to produce plastics, resins, dyes, rubbers, detergents, lubricants, synthetic fibers, and many more.
Benzene can be produced through petroleum reforming, dealkylation (removing alkyl groups from benzene), and isolating benzene rings from gas tar. However, this compound is highly toxic, so each process must be carried out with great care.
This compound was discovered around 200 years ago and is used in various industries. Statista notes that in 2022, the global benzene market volume reached 60.28 million metric tons. By 2030, this amount is expected to have increased to 70.04 million metric tons.
Furthermore, benzene can also react with many substances. For example, benzene can react with sulfuric acid and nitric acid to form nitrobenzene through a process called nitration.
Then, benzene can react with hydrogen in the hydrogenation process or attach water molecules to the benzene ring. Then, benzene can react with iron bromide in the bromination process, where the benzene structure will change because the hydrogen atoms are converted to bromine atoms.
Read also: 17 Household Chemical Products and How to Store Them Safely
Uses of Benzene

As a building block for other chemicals, benzene undergoes a reaction with other chemical compounds to produce new products. For example, benzene is a feedstock for creating ethylbenzene. Ethylbenzene is used to make styrene, which is used to manufacture polystyrene.
Regarding ethylbenzene, Chandra Asri Group, as a leading chemical solution company in Southeast Asia, provides ethylbenzene with a purity of more than 99.7%. You can use it for making some byproducts, such as styrene monomer.
Naturally, benzene comes from crude oil. The oil is processed into gasoline, jet fuel, petroleum products, diesel, solvents, lubricants, and so on. That’s why these products contain at least a bit of benzene in them.
In addition to fuels, benzene is also used to produce other industrial products, such as:
- Plastic.
- Foam.
- Dyes.
- Detergent.
- Solvent.
- Insecticides and pesticides.
- Polystyrene.
- Nylon.
- Epoxy resin.
- Polyester fiber.
- Cleaning products.
- Adhesive.
- Paint.
- Rubber.
- Certain medicines.
- Lubricants.
- Explosives.
- And many more.
Read also: 9 Petroleum Products You Must Know, from Gasoline to Naphtha
Benzene’s Byproducts
The benzene’s byproducts are made of hydrogen atom substitution with other components. Here are some byproducts you should know:
1. Phenol
Phenol is benzene’s byproduct used to manufacture pesticides, disinfectants, plastics, and phenolic resins. Phenol comes from the substitution of one hydrogen atom with a hydroxyl group.
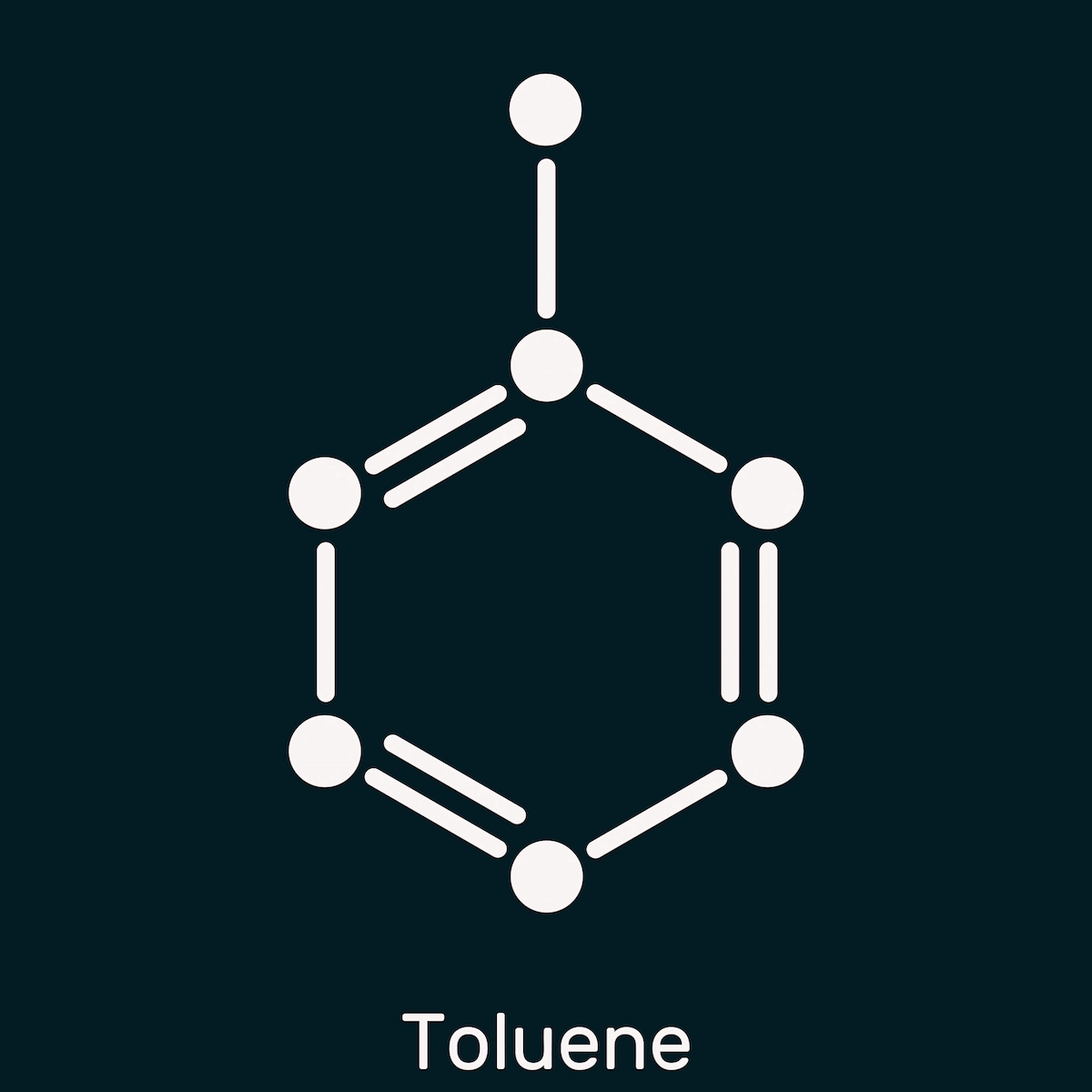
2. Toluene
Toluene is utilized to produce polyurethanes, industrial solvents, and trinitrotoluene (TNT) for explosives. You can get toluene by substituting one hydrogen atom with a methyl group.
3. Nitrobenzene
Nitrobenzene is used to create solvents and anilines. It originates from a nitration reaction, where a nitro group replaces a hydrogen atom.
4. Benzoic Acid
Benzoic acid is used as a food preservative. It has a carboxylate group bound to a benzene ring.
5. Aniline
Aniline is usually utilized to create dyes and rubbers. It results from the substitution of an amino group for one hydrogen atom.
6. Cyclohexane
Cyclohexane undergoes benzene hydrogenation using a nickel-based catalyst. It is used to produce nylon and lubricant oils.
7. Cumene
Cumene, or isopropylbenzene, comes from the addition of an isopropyl group to benzene. Cumene is used to create enamel and paint solvent.
8. Ethylbenzene
Ethylbenzene comes from the addition of an ethyl group to benzene. It is a feedstock to manufacture styrene monomer.
From the explanation above, it can be concluded that benzene is one of the crucial feedstocks for industries. The byproducts become the building block for other products, one of which is ethylbenzene for making styrene monomer.
If your company is looking for a high-quality ethylbenzene, entrust it to Chandra Asri Group. As #YourGrowthPartner, we provide various chemical compounds, including those for plastics, rubbers, and oils.
Not only Chandra Asri Group, but you can also rely on Aster, our subsidiary based in Singapore. Aster is an energy solution built on the acquisition of Chandra Asri Group and Glencore by Shell Energy and Chemicals Park (SECP).
This chemical and energy complex consists of an oil refinery with a capacity of 237,000 barrels per day, an ethylene cracker with a capacity of 1.1 million metric tons per year, and other downstream chemical assets.
Therefore, entrust your chemical needs to energy, chemical, and infrastructure solution companies like Chandra Asri Group and Aster!
Read also: What Is Plastic Made of? Here Are the Materials & Process
.png&w=3840&q=75)
.png&w=3840&q=75)